Table of Contents >> Show >> Hide
- Monoclonal antibodies, explained without a lab coat
- Two very different monoclonal-antibody stories in COVID-19
- How anti-SARS-CoV-2 monoclonal antibodies work
- A quick timeline: from game-changer to “benched by variants”
- So… are monoclonal antibodies still used for COVID-19 in the U.S. today?
- What to expect if you’re getting a monoclonal antibody infusion
- Hospital use: monoclonal antibodies that calm the immune system
- Monoclonal antibodies vs. antivirals vs. vaccines: who does what?
- Common questions (and myths) people have
- The future: will monoclonal antibodies make a comeback for treatment?
- Real-world experiences: what people often notice (and what surprised them)
- Bottom line
- SEO tags
If antibodies are your immune system’s “wanted posters,” then monoclonal antibodies are the professionally printed, high-resolution versionslaminated, color-corrected, and delivered overnight.
They’re lab-made proteins designed to recognize a specific target. For COVID-19, that target is often (but not always) something related to the virus or the body’s inflammatory response.
And here’s the twist: monoclonal antibodies (often shortened to “mAbs”) have had a very dramatic COVID-era storylinemore “limited series with plot twists” than “boring textbook chapter.”
In this guide, we’ll break down what monoclonal antibody treatment is, how it was used for COVID-19, what it can and can’t do today, and what the experience is like in real life.
We’ll keep it practical, accurate, and just humorous enough that your brain won’t try to file for a nap.
Monoclonal antibodies, explained without a lab coat
Antibodies 101
Antibodies are proteins your immune system makes to recognize and latch onto “not-you” things (like viruses). When an antibody binds to a virus, it can:
- Block the virus from entering cells (neutralization).
- Tag the virus so other immune cells can clean it up (a.k.a. “Hey team, over here!”).
Monoclonal vs. polyclonal (why the “mono” matters)
Your body naturally makes a mix of antibodies against different parts of a virus. That mix is “polyclonal.”
A monoclonal antibody is a single, specific antibody cloneengineered to bind one target really well.
Think of it like a key cut for one lock, instead of a jangly keyring full of “maybe this one?” options.
Two very different monoclonal-antibody stories in COVID-19
When people say “monoclonal antibody treatment for COVID-19,” they might mean one of two categories:
1) Anti-SARS-CoV-2 neutralizing antibodies (virus-targeting)
These are designed to bind the virus (often the spike protein) and prevent it from infecting cells. Earlier in the pandemic, several were authorized for:
- Early outpatient treatment for high-risk people (to reduce hospitalization risk).
- Pre-exposure prophylaxis for certain immunocompromised individuals (prevention).
2) Immunomodulatory antibodies (inflammation-targeting)
These don’t “attack the virus” directly. Instead, they help manage an overactive immune response in severe or critical illnessgenerally in hospitalized patients, alongside other treatments.
How anti-SARS-CoV-2 monoclonal antibodies work
SARS-CoV-2 infects cells by using its spike protein to attach to receptors on human cells. Many COVID-19 neutralizing monoclonals are designed to bind parts of spike so the virus can’t “dock.”
If the virus can’t get inside cells efficiently, it has a harder time multiplying, and the illness is less likely to escalate.
Timing matters because neutralizing antibodies work best when viral replication is still ramping uptypically early in the course of illness.
Once the disease is driven more by inflammation than active viral growth, the benefit of virus-targeting antibodies drops off.
A quick timeline: from game-changer to “benched by variants”
Early in the pandemic, monoclonal antibodies were a big deal for outpatient careespecially before vaccines and widely available oral antivirals.
But SARS-CoV-2 kept mutating. Many monoclonals were highly specific to older versions of spike, so newer variants gained mutations that made those antibodies bind poorly (or not at all).
Over time, multiple monoclonal antibody Emergency Use Authorizations (EUAs) were paused, limited by region, or revoked as susceptibility changed and products expired.
This is not because the idea of monoclonals “failed”it’s because the virus is a moving target and monoclonals can be very target-specific.
So… are monoclonal antibodies still used for COVID-19 in the U.S. today?
Outpatient “treatment” monoclonal antibodies: largely not available (for now)
As of recent federal guidance and updates, neutralizing monoclonal antibodies are not a routine option for treating mild-to-moderate COVID-19 in outpatients the way they were earlier in the pandemic.
Current outpatient treatment strategies generally focus on antivirals (like nirmatrelvir/ritonavir or remdesivir) rather than monoclonals.
Pre-exposure prophylaxis: one notable monoclonal option for certain people
There is an FDA-authorized monoclonal antibody for pre-exposure prophylaxis (prevention) in certain people who are moderately to severely immunocompromised:
pemivibart (brand name Pemgarda).
It is intended for people who are unlikely to mount an adequate immune response to vaccination and who meet the authorization criteria.
Key practical details about pemivibart (Pemgarda):
- Who: Adults and adolescents 12+ who weigh at least 40 kg (about 88 lbs), who are moderately/severely immunocompromised and meet authorization criteria.
- What it’s for: Pre-exposure prophylaxis (extra layer of prevention), not a substitute for vaccination.
- What it’s not for: It is not authorized to treat active COVID-19 and not authorized for post-exposure prophylaxis.
- How it’s given: IV infusion over a minimum of 60 minutes.
- How often: An initial 4500 mg dose, then 4500 mg about every 3 months (timed from the most recent dose), per authorization materials.
- Safety reality check: Because allergic reactions can occur, it should be given where staff can treat anaphylaxis and activate emergency services if needed.
- Vaccines + Pemgarda timing: If you recently got a COVID-19 vaccine, Pemgarda is administered at least 2 weeks after vaccination.
- Variants matter: Authorization includes variant-susceptibility considerations; effectiveness can change if the virus changes.
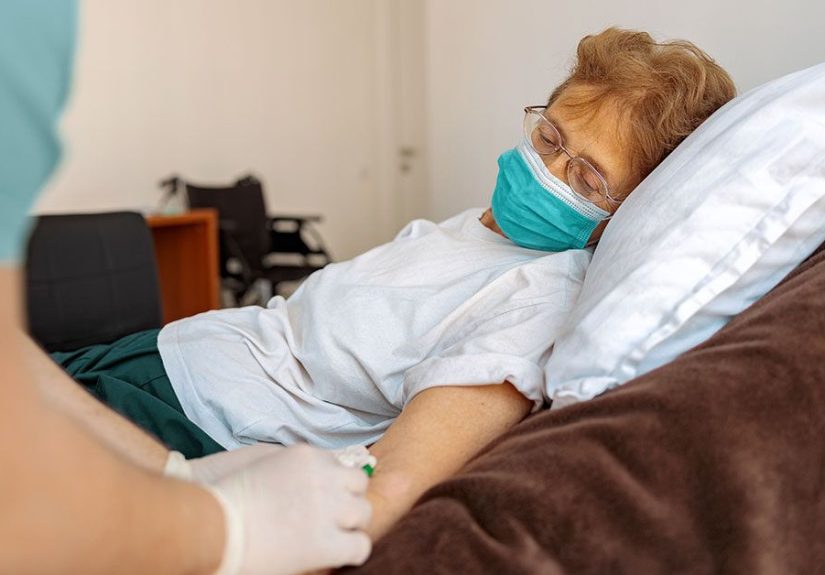
What to expect if you’re getting a monoclonal antibody infusion
Even if you’ve never had an infusion before, the experience is usually more “calm clinic visit” than “TV medical drama.”
(No one bursts through the doors yelling “We’re losing them!”and if they do, that’s probably a different department.)
Before the appointment
- Eligibility check: Your clinician confirms you meet the criteria (for example, immunocompromised status for pre-exposure prophylaxis).
- Medication review: They’ll ask about allergies and relevant medical history, and they may discuss vaccine timing.
- Scheduling: Infusion centers often book in time blocks because of monitoring requirements.
During the infusion
- IV placement: A small catheter goes into a vein (usually in your arm).
- Infusion time: For Pemgarda, the infusion is at least 60 minutes.
- Observation: Many centers monitor you during and after for potential reactions.
Afterward
Many people feel fine and head home. Some may have mild side effects (think: headache, fatigue, nausea, infusion-site discomfort).
Severe allergic reactions are uncommon but taken seriously, which is why the setting and monitoring matter.
Hospital use: monoclonal antibodies that calm the immune system
When COVID-19 becomes severe or critical, the problem may shift from “virus multiplying” to “immune system overreacting.”
In that setting, clinicians may use medications that reduce harmful inflammationsometimes including monoclonal antibodies.
Tocilizumab (and similar immunomodulators)
Tocilizumab is a monoclonal antibody that targets the IL-6 receptor (IL-6 is involved in inflammation).
Guidelines have recommended immunomodulators such as tocilizumab (often alongside systemic corticosteroids) in certain hospitalized patients with rapidly progressing severe or critical COVID-19 who need additional immune modulation.
This is not a DIY decisionthese drugs are used based on severity, oxygen needs, lab markers, and clinician judgment.
Other monoclonal approaches in critical illness
Some guideline updates also discuss specialized monoclonal antibodies for critically ill patients (for example, targeting inflammatory pathways beyond IL-6).
These recommendations can be narrow and evolve as evidence grows, so hospitals typically follow updated clinical guidelines and local protocols.
Monoclonal antibodies vs. antivirals vs. vaccines: who does what?
It helps to think of COVID-19 tools like a layered defense system:
- Vaccines: Train your immune system ahead of time. They lower the risk of severe disease for most people.
- Antivirals: Interfere with viral replication after infectionbest when started early.
- Neutralizing monoclonal antibodies: Provide ready-made virus-targeting antibodies (helpful early, but highly dependent on variant susceptibility).
- Immunomodulatory monoclonals: Reduce harmful inflammation in severe cases, usually in the hospital.
That’s also why you may hear clinicians emphasize: “Call early if you’re high-risk.”
Early treatment windows matter most for antiviralsand historically mattered most for outpatient neutralizing monoclonals, too.
Common questions (and myths) people have
“If monoclonals exist, why bother with vaccination?”
Because monoclonals are not a universal, permanent shield. Their effectiveness can change with variants, and they’re typically reserved for specific situations.
Vaccination remains a foundational strategy for preventing severe outcomes for most people.
“Are monoclonals like antibiotics?”
Nope. Antibiotics target bacteria. Monoclonals are proteins that bind specific targetslike viral components or immune molecules.
“Will a monoclonal antibody make me test positive?”
A monoclonal antibody is not the virus, and it doesn’t create an infection.
COVID-19 viral tests detect viral genetic material or viral proteins, not the presence of therapeutic antibodies.
“Can I just ask for monoclonals instead of antivirals?”
In the U.S., current outpatient treatment choices are usually driven by what is authorized, available, and effective against circulating variants.
That means antivirals are often the main outpatient option, while monoclonals may be limited to specific preventive use in immunocompromised people.
The future: will monoclonal antibodies make a comeback for treatment?
Researchers continue to pursue antibodies that are:
- More broadly neutralizing across many variants
- Longer-acting (so protection lasts months)
- Easier to administer (fewer logistical hurdles)
The big challenge is speed: the virus evolves quickly, and it takes time to develop, test, and authorize new products.
But the concept remains powerfulespecially for people who can’t rely on a strong vaccine response.
Real-world experiences: what people often notice (and what surprised them)
Let’s talk about the human sidebecause “4500 mg IV over 60 minutes” is accurate, but it doesn’t capture what it feels like to actually go through it.
Here are common experiences people report when monoclonal antibodies are part of their COVID-19 prevention or care plan.
The emotional whiplash: relief mixed with “Wait, am I allowed to relax?”
Immunocompromised patients often describe living with constant mental math: “Is this restaurant too crowded?” “Can I visit family?” “Will I be the one who gets really sick?”
When a preventive monoclonal option is added, many people feel a real sense of relieflike someone finally handed them an extra umbrella in a storm.
But it can also bring a new kind of anxiety: “What if variants change again?” “What if this stops working?”
The best mindset tends to be: layered protection.
People who feel most confident often combine vaccination (when recommended), masking in high-risk settings, and early testing plansso the monoclonal isn’t carrying the whole team on its back.
The logistics: “I didn’t realize prevention could involve an infusion appointment”
A lot of folks assume prevention is always a pill or a shot.
The infusion-center reality can be surprisingly… normal. You check in, sit in a recliner, scroll your phone, and try not to make awkward eye contact with the IV pole.
Some bring a hoodie, headphones, and a snackbasically treating it like a very quiet layover at an airport, minus the overpriced pretzels.
The most common “I wish someone told me” tips:
- Wear sleeves that roll up easily (your fashion moment can wait an hour).
- Hydrate beforehand (easier IV starts are everyone’s love language).
- Plan low-key time after, just in case you feel tired.
Side effects: most people feel okay, but the monitoring feels intense
Many people report minimal side effectsmaybe a headache or fatigue that blends into the background noise of modern life.
What stands out more is how seriously clinics take allergic reactions.
Staff may ask repeated questions about symptoms and keep you a bit longer for observation.
Some patients interpret that as “Oh no, this must be dangerous,” when it’s often the opposite: it’s a sign the clinic is prepared and following safety protocols.
If you’ve ever watched a flight attendant demonstrate a seatbelt for the thousandth time, it’s like that: boring, repetitive, and exactly what you want in an emergency.
The confidence boost: having a plan beats doom-scrolling
The most consistent theme people describe is that having a clear plan lowers stress.
That plan might be: “I’m vaccinated; I have Pemgarda; I’ll test early if symptoms start; and I know who to call about antiviral eligibility.”
It’s not invincibility. But it replaces vague fear with steps.
And in healthcare, that’s often the difference between feeling powerless and feeling prepared.
Bottom line
Monoclonal antibodies are lab-made antibodies that can either target the virus (neutralizing antibodies) or calm harmful inflammation (immunomodulators).
For COVID-19 in the U.S., the role of monoclonals has changed over time:
outpatient treatment monoclonals largely fell out of use as variants reduced effectiveness, while a preventive monoclonal option (pemivibart/Pemgarda) exists for certain immunocompromised people.
In hospitals, certain monoclonal antibodies may still play a role in severe or critical disease as part of a broader treatment strategy.
If you or someone you care for is high-risk, the most useful move is not picking a “favorite” therapyit’s building a timely, layered plan with a clinician:
prevention strategy, rapid testing, and early treatment options when appropriate.